New MIT Study: Nanoparticles Can Clean up Chemicals, BPA, Pesticides, from Soil, Water
(EnviroNews World News) — An accidental discovery made by medical researches has yielded a “quick” and “easy” way to remove toxic chemicals from the environment MIT News has reported.
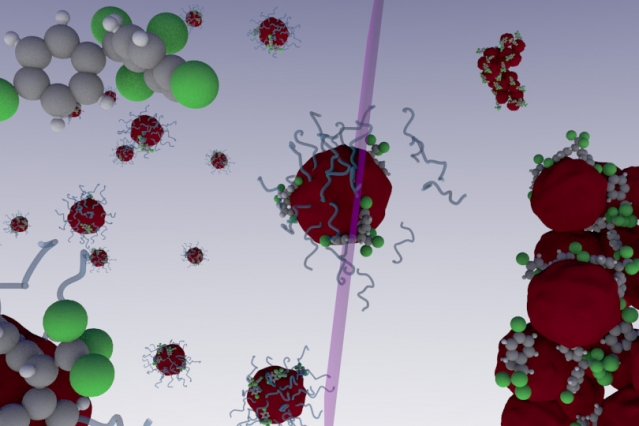
In a paper published in Nature Communications, scientists from MIT and the Federal University of Goiás in Brazil revealed how a nano-sized polymer in tandem with ultra violet (UV) light can isolate, and ultimately eliminate, myriad non-water soluble toxins from either water or soil.
Ferdinand Brandl and Nicolas Bertrand, the paper’s two lead authors, both have backgrounds in pharmacology, and made the accidental discovery when attempting to create nanoparticles that would carry drugs into cancer cells. The pair said finding the particles could remediate environmental contamination came as a “happy accident,” reported MIT News.
“The researchers synthesized polymers from polyethylene glycol, a widely used compound found in laxatives, toothpaste, and eye drops and approved by the Food and Drug Administration as a food additive, and polylactic acid, a biodegradable plastic used in compostable cups and glassware,” wrote MIT News.
Polymers are often composed of many molecular monomers that compose a much larger macromolecule, and can be made of either organic or non-organic material. The ones used by Brandl and Bertrand have a hydrophobic internal core, while maintaining a hydrophilic outer shell. Since many toxins like Bisphenol A (BPA) are also hydrophobic as well, meaning they don’t mix with water, they are attracted to the hydrophobic core in the polymers. But, when they hit they hydrophilic outer shell, they absorb into it and become “trapped.”
They nanopolymers are then exposed to UV light which causes the toxin-saturated particles to aggregate, or clump together in large masses. The clods, along with the sequestered chemicals, are then removed. What’s more, only a very small amount of the particles are necessary to eliminate a relatively large amount of toxins.
UV light is already used to purify specific substances from tainted water in some wastewater treatment plants. This is what the authors said inspired their idea with the nanomaterials because one of them had already created another type of polymer that splits apart on contact with UV light — the opposite of what this material does.
According to the research, the scientists were able to extract and remove phthalates from wastewater, BPA from printing paper, and polycyclic aromatic hydrocarbons (PAHs) from contaminated soil. Since the dawn of the petrochemical age, those substances have been accumulating in drinking-water supplies, soil and human and embryonic tissue — and all are known by science to be hormone disrupting carcinogens.
The authors iterated the process is irreversible once clumping has occurred, and said the polymers were highly biodegradable. “The fundamental breakthrough, according to the researchers, was confirming that small molecules do indeed adsorb passively onto the surface of nanoparticles,” writes MIT News.
“The interactions we exploit to remove the pollutants are non-specific,” said Brandl. “We can remove hormones, BPA, and pesticides that are all present in the same sample, and we can do this in one step.”
These polymers are manufactured at room temperature and require no special preparation to attract to, or bond with any particular compound. They are widely applicable and can attach to a wide array of hydrophobic substances, offering a plethora of potential applications from environmental cleanup to medical procedures. MIT’s article even suggested the nanos could be used to eliminate organic solvents in processes such as “decaffeinating coffee and making paint thinners.”
EnviroNews has been aware of the existence of technology to remediate environmental pollution with nanoparticles for some time. In 2012 a film was released by researchers, again at MIT, that demonstrates the cleanup of oil from water by the infusion of a magnetically charged liquid nanoparticle solution that is attracted to, and bonds with oil, whereafter the nanos, along with the oil, are magnetically recovered and removed from the water. In this process the oil can actually later be separated from the nano solution and sent to a refinery for processing.
In China hundreds of entire river ecosystems have been killed from chemical dumping — in America countless superfund sites have been designated due to industrial tailings and reckless spillage into soil and water. The stark and ever-growing sum of tarnished sites and waterways has us watching the world of nano technology scrupulously to see what other interesting materials will be designed with the intention of scavenging deadly and mutagenic poisons from the environment.
FILM AND ARTICLE CREDITS
- Emerson Urry - Journalist, Author


![Leading the Charge for America’s Wild Horses on Capitol Hill: NBA/NFL Celeb. Bonnie-Jill Laflin: ‘[Politics] won’t stop us from fighting’](https://cf-images.us-east-1.prod.boltdns.net/v1/static/1927032138001/f46b2158-cead-47f0-ab44-4b027059411a/4e4afcf2-937d-4a9d-acba-1b82e2efd4c6/160x90/match/image.jpg)


